Click the thumbnail to expand to full-screen view.
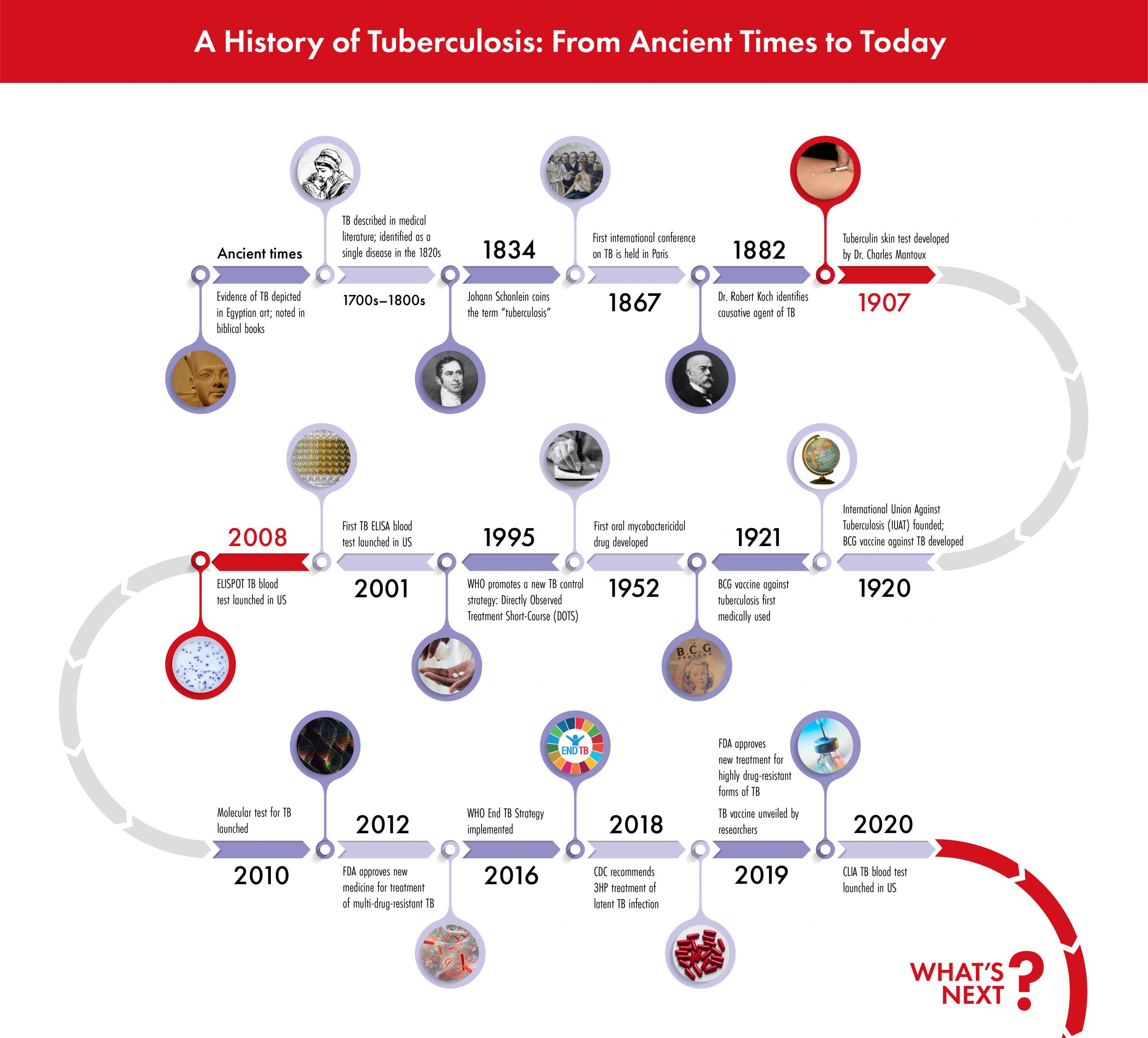
Here is a brief look at the history of TB testing methods, highlighting the significant milestones for advancements in technology and clinical practice.
Tuberculosis, or TB, is an ancient human disease that remains a major cause of death today. It is one of the most challenging public health problems worldwide. In 2014, the World Health Organization (WHO) set forth the End TB strategy: this initiative aims to end the global TB epidemic, with targets to reduce TB deaths and new cases incrementally over several milestones in 2020, 2025, and 2030.
Focusing resources on at-risk and under-served populations is an important part of the fight to end TB, and understanding the history of TB testing methods is an important foundation for advancements in public health care and disease management.
References
Annals of the American Thoracic Society. Treatment of tuberculosis. A historical perspective. www.atsjournals.org/doi/full/10.1513/AnnalsATS.201509-632PS. Accessed November 12, 2019.
BBC News. “Game changing” tuberculosis vaccine a step closer. www.bbc.com/news/world-asia-india-50205762. Accessed November 12, 2019.
Cave AJE, Demonstrator A. The evidence for the incidence of tuberculosis in ancient Egypt. British Journal of Tuberculosis. 1939;33(3):142-152.
CDC. History. World TB day. TB. www.cdc.gov/tb/worldtbday/history.htm. December 13, 2018. Accessed November 11, 2019.
CDC. 3HP for latent TB infection treatment. www.cdc.gov/nchhstp/newsroom/2018/treatment-of-latent-TB-infection.html. November 21, 2018. Accessed November 12, 2019.
FDA. Office of the Commissioner. FDA approves new drug for treatment-resistant forms of tuberculosis that affects the lungs. www.fda.gov/news-events/press-announcements/fda-approves-new-drug-treatment-resistant-forms-tuberculosis-affects-lungs. September 11, 2019. Accessed November 12, 2019.
Lawn SD, Nicol MP. Xpert® MTB/RIF assay: Development, evaluation and implementation of a new rapid molecular diagnostic for tuberculosis and rifampicin resistance. Future Microbiol. 2011;6(9):1067-1082.
Lempp JM, Zajdowicz MJ, Hankinson AL, et al. Assessment of the QuantiFERON-TB Gold In-Tube test for the detection of Mycobacterium tuberculosis infection in United States Navy recruits. PLoS One. 2017;12(5):e0177752.
Mahajan R. Bedaquiline: First FDA-approved tuberculosis drug in 40 years. Int J Appl Basic Med Res. 2013;3(1):1-2.
McLaren ZM, Milliken AA, Meyer AJ, Sharp AR. Does directly observed therapy improve tuberculosis treatment? More evidence is needed to guide tuberculosis policy. BMC Infectious Diseases. 2016;16(1):537.
Nayak S, Acharjya B. Mantoux test and its interpretation. Indian Dermatol Online J. 2012;3(1):2-6.
Oxford Immunotec North America. T-SPOT®.TB Gains FDA Premarket Approval. www.oxfordimmunotec.com/north-america/news/t-spot-tb-gains-fda-premarket-approval/. Accessed November 12, 2019.
Sandhu GK. Tuberculosis: Current situation, challenges and overview of its control programs in India. J Glob Infect Dis. 2011;3(2):143-150.
The Union. History. www.theunion.org/what-we-do/conferences/world-conference-on-lung-health/history. Accessed November 11, 2019.
The Union. History. www.theunion.org/who-we-are/history. Accessed November 11, 2019.
WHO. WHO targets elimination of TB in over 30 countries. www.who.int/mediacentre/news/releases/2014/tb-elimination/en/. Accessed November 12, 2019.